Source: Next Steps in Derm
What is Hyperhidrosis (HH)?
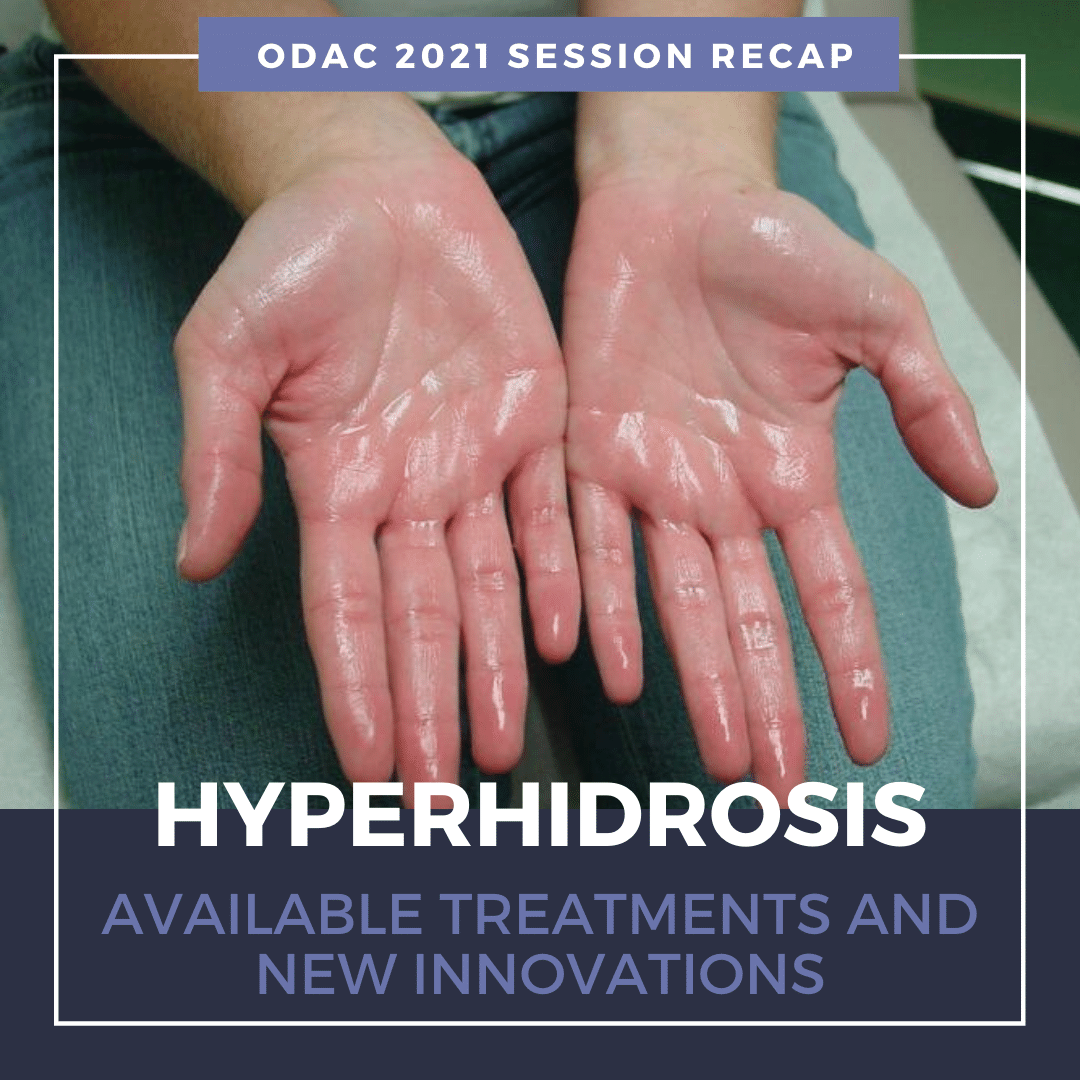
Primary HH is defined as excessive, bilateral, and generally symmetrical sweating at abnormal levels unprompted by activity or environment.
Hyperhidrosis is characterized by abnormal sweating beyond what is needed for thermoregulation. It is not a normal physiologic response. Nearly 15 million people in the United States suffer from hyperhidrosis and half of those individuals have axillary hyperhidrosis. HH peaks in adulthood and approximately two-thirds of these individuals have never discussed their primary axillary hyperhidrosis with a healthcare professional.
Why aren’t more patients seeking help for their abnormally excessive sweating?
Many of these patients do not realize their excessive sweating is a medical condition and are unaware of protentional treatment options. On average, patients wait at least 10 years before seeking medical help for their abnormally excessive sweating1.
Patients with hyperhidrosis are reluctant to seek medical care, despite the negative impact on their quality of life.
Primary versus Secondary Hyperhidrosis
Primary Focal Hyperhidrosis is defined as excessive focal sweating of at least 6 months duration without any obvious cause with at least two of the following features:
-
- Onset before age 25, often as early as 9 years old
- A bilateral and symmetric pattern
- Impairment of daily activities
- Occurring at least once per week
- Cessation during sleep
- A positive family history
90% of all diagnosed cases of HH are primary and two-thirds of all HH patients report a positive family history.
Secondary Generalized Hyperhidrosis can be caused by or associated with another medical condition or results as a side effect of a medication. It usually presents with large areas of the body affected. Patients with secondary HH may experience sweating even when they are sleeping. It is important to differentiate between primary and secondary hyperhidrosis. Review the patient’s medical history and review of systems should point to any secondary causes. Patients who do not fit the classic pattern of primary hyperhidrosis should undergo further evaluation, and may need laboratory studies or radiographic evaluation.
Can the coronavirus spread through sweat?
There is no evidence that Covid-19 can be transmitted through sweat. The virus is transmitted by respiratory droplets. Dr. Adam Friedman notes that sweat could actually help prevent the transmission of Covid-19 due to the inherent antimicrobial activity of sweat. For anyone worried about transmission of the virus, it’s important to emphasize key tenets voiced from experts:
-
- Wash/sanitize your hands often and well
- Avoid touching your face
- Wear a mask in public
- Maintain physical distance, about 6 feet, from others
- Sanitize frequently-touched surfaces
- Follow your community’s public health recommendations
For more information, visit the International Hyperhidrosis Society’s (IHS) website: www.sweathelp.org
IHS is the only independent, non-profit, global organization that strives to improve quality of life among those affected by hyperhidrosis.
Treatment of Hyperhidrosis
A wide range of techniques has been utilized in the treatment of HH. First-line therapy for HH is topical formulations. Over-the-counter antiperspirants are usually not strong enough to significantly improve excessive sweating. Prescription aluminum salts are usually the first-line treatment for HH. The aluminum salts act as a plug, precipitating and blocking the sweat ducts. Sweating is reduced in the evening. Thus, application to dry body surfaces at night is ideal. Non-medicated deodorant can be applied in the morning after bathing. The fragrance from deodorants can be used to mask body odor from the microbiome of the skin.
Topical Aluminum Pearls:
-
- Aluminum salts must remain on the skin for 6 – 8 hours to be effective
- Overnight application is recommended
- Wash treated skin in the morning before sweating begins
- Wait 24 to 48 hours after shaving
- Beware of damage to fabrics
- A reaction occurs when aluminum mixes with the salts of your sweat, which may cause yellow stains on white fabrics.
- Irritated skin can be treated with low potency topical steroid
Topical antiperspirants are just not that effective for hyperhidrosis.
While over-the-counter products are most commonly recommended, they offer the least patient satisfaction. Topical antiperspirants are safe and simple to use. However, application is time-consuming, only provides temporary relief, and may cause skin irritation at the application site.
Fortunately, there are novel topical therapies available to patients with excessive underarm sweating, or axillary hyperhidrosis. Topical glycopyrronium tosylate (GT) wipes are FDA approved for treatment of axillary HH. This is the first and only prescription cloth towelette approved to treat excessive underarm sweating. GT works by blocking receptors responsible for sweat gland activation1. A significant number of patients can see improvement as early as 1 week. After 4 weeks of treatment in the clinical trials, GT resulted in substantially greater improvements compared to the control group. Patients were happy with the fast onset of effect and reported overall improved quality of life2:
-
- No longer avoiding interactions with other people
- No longer showering/bathing more than once a day or changing shirts during the day
- Feeling less embarrassed or confident
- No longer avoiding activities for work or hobby
Topical Glycopyrronium Tosylate Pearls:
-
- Approved for use in children ≥9 and adults
- Applied nightly to clean axillary skin
- Use 1 towelette by wiping across each entire underarm once
- Wash hands thoroughly after use to avoid inadvertent transfer of the drug to areas such as the eyes
- No need to remove hair or occlude the area for use
- May use OTC antiperspirants as needed
- Most patients see improvement by 2-3 weeks
- May hold dose as needed
Dr. Friedman notes that use of topical GT for treatment of palmar/plantar hyperhidrosis is off-label. He instructs his patients to rub the towelette between hands until dry, apply Aquaphor, and then wear gloves for at least 1 hour, in order to achieve clinical benefits.
It is important to monitor for anticholinergic side effects. The most common reported adverse effects include dry mouth and mydriasis. Use of gloves with the towelette and careful handwashing can help mitigate anticholinergic side effects. GT is non-invasive, effective, and well-tolerated. There is minimal irritation and can be safely used in pediatric populations. Aside from long-term therapy and potential for cost issues, topical GT provides clinically meaningful benefits for patients with primary axillary hyperhidrosis.
Iontophoresis
Iontophoresis is a process of transdermal drug delivery by use of a voltage gradient on the skin. It is often recommended for patients who have failed topical therapies or in patients with HH of the palms and soles. During iontophoresis, a medical device is used to pass a mild electrical current through water and through the skin’s surface1. Aluminum chloride or anticholinergics can be added to the water to help draw electric current. This non-surgical method is painless and effective. However, it requires long-term, weekly therapy to maintain efficacy. Of note, iontophoresis is contraindicated in patients with pacemakers or implants, or during pregnancy.
Microwave device
Non-invasive microwave technology uses thermal energy to ablate sweat glands. The device is FDA approved for treatment of severe axillary HH. The treatment is performed in a physician’s office with local anesthesia. Patients typically require 1-3 treatments to achieve long-lasting results. There is little to no discomfort during the procedure. Side effects are minor and include underarm swelling, redness, and tenderness lasting for several days. Numbness and tingling can occur in the upper arm or armpit and may last for about 5 weeks1.
Systemic Therapies
There are no systemic agents FDA approved for treatment of HH. Most data for systemic treatment comes from case reports or small case series. Systemic treatment may be more helpful in multifocal hyperhidrosis or hyperhidrosis of spinal cord injury. The two most common prescribed oral agents include Glycopyrrolate and Oxybutynin.
Glycopyrrolate does not cross the blood-brain barrier, resulting in less CNS effects. There is also an oral solution available for pediatric populations. Oxybutynin is safe in children as well. These therapies are cost-effective, but long-term therapy is required.
-
- Adverse effects due to Anticholinergic properties:
- Dry mouth
- Dry eyes
- Constipation
- Blurred vision
- Difficulty with urination
There is a risk of hyperthermia in pediatric patients taking systemic anticholinergics. It is important to watch for behavioral changes, as well as drowsiness, dizziness and confusion. Despite the efficacy, most patients discontinue therapy due to anticholinergic side effects.
Other systemic therapies include beta-blockers and Clonidine. Beta-blockers can be used for social phobias/performance anxiety. However, it is contraindicated in patients with bradycardia, AV block, or asthma. Of note, beta-blockers are a common cause of drug-induced psoriasis or exacerbation of psoriasis. Consider other treatment options in patients suffering from both psoriasis and HH. Clonidine has been evaluated in small case series. It is a great medication in treating patients with both hyperhidrosis and flushing.
Minimally invasive approaches
OnabotulinumtoxinA is FDA approved for axillary hyperhidrosis unresponsive to other therapies. It prevents release of acetylcholine from the neuromuscular junction. Injection technique involves a grid pattern (mapped via surgical marker) using a 30-gauge needle. Injection depth is approximately 2 mm and at a 45º angle to the skin surface with bevel side up. Injections are spaced 1-2cm apart for a total of 50 units injected per axilla.
Read more…..