Source: Next Steps in Derm
This information was presented by Dr. Adam Friedman at the 16th Annual ODAC Dermatology, Aesthetics and Surgical Conference held January 18th-21st, 2019 in Orlando, FL. The highlights from his lecture were written and compiled by Dr. InYoung Kim.
If you’re a coffee drinker, you may be relieved to know that there was an inverse association between caffeine intake and risk of rosacea in a recent study. That was a huge relief for me for sure! Unfortunately, we can’t prescribe caffeine for rosacea and call it a day. So, what works?
High-yield pearls on the pathophysiology and management of rosacea are shared by Dr. Adam Friedman – Professor and Interim Chair of Dermatology, Residency Program Director, Director of Translational Research, and Director of the Supportive Oncodermatology Clinic in the Department of Dermatology at The George Washington University School of Medicine & Health Sciences. Here are the highlights.
New Approach to Diagnosis and Categorization of Rosacea
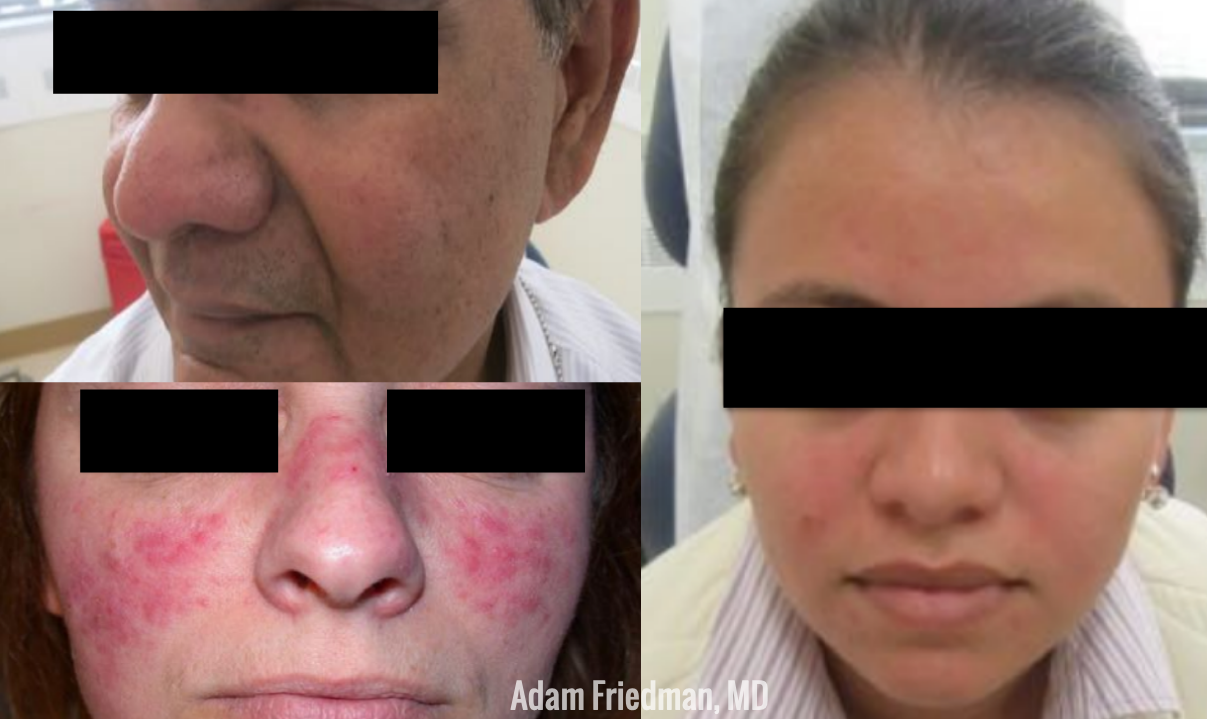
First, let’s talk about diagnosis. Rather than categorizing into 4 classic subtypes that we learned in the textbook, rosacea may be better defined by “phenotypes”. Diagnostic phenotypes include 1) having fixed centrofacial erythema in a characteristic pattern that may periodically intensify or 2) phymatous changes. In the absence of these, the presence of 2 or more major features may be diagnostic, including papules/pustules, flushing, telangiectasia, ocular symptoms. Some secondary phenotypes that may help with diagnosis are burning/stinging, edema, dry appearance, and ocular rosacea.
Rosacea in Skin of Color – Rosacea Does Not Discriminate!
While rosacea has widely been considered a disorder selectively affecting the Caucasian population, this is not true! Perhaps due to this bias, delayed diagnosis has been reported in substantial numbers. In fact, the prevalence of rosacea in skin of color is as high as 10%! That is significant. Please spread the word! So how do they present differently than Caucasian patients? While you may not see the persistent facial erythema (which is common in whites), the granulomatous subtype and papules/pustules are more common in skin of color. Asking about the secondary phenotypes noted above (burning/stinging, edema, dry appearance, and ocular rosacea) may also be helpful in diagnosis.
Therapeutic Options – Combo is King!
While many prescription treatment options exist (outlined below), patient education concerning proper general skin care is of utmost importance. Make sure to include these in your counseling: daily sunscreen, gentle moisturizers, gentle cleansers, avoid triggers.
A list of FDA-approved topical therapies that you may choose from:
- Azelaic acid (15% gel/foam)
- Metronidazole (0.75% and 1%)
- Sodium sulfacetamide 10% and sulfur 5%
- Brimonidine (0.33% gel)
- Ivermectin 1%
- Oxymetazoline (1% cream)
What would a typical daily plan look like for a moderate-to-severe rosacea patient? Here are Dr. Friedman’s tips: