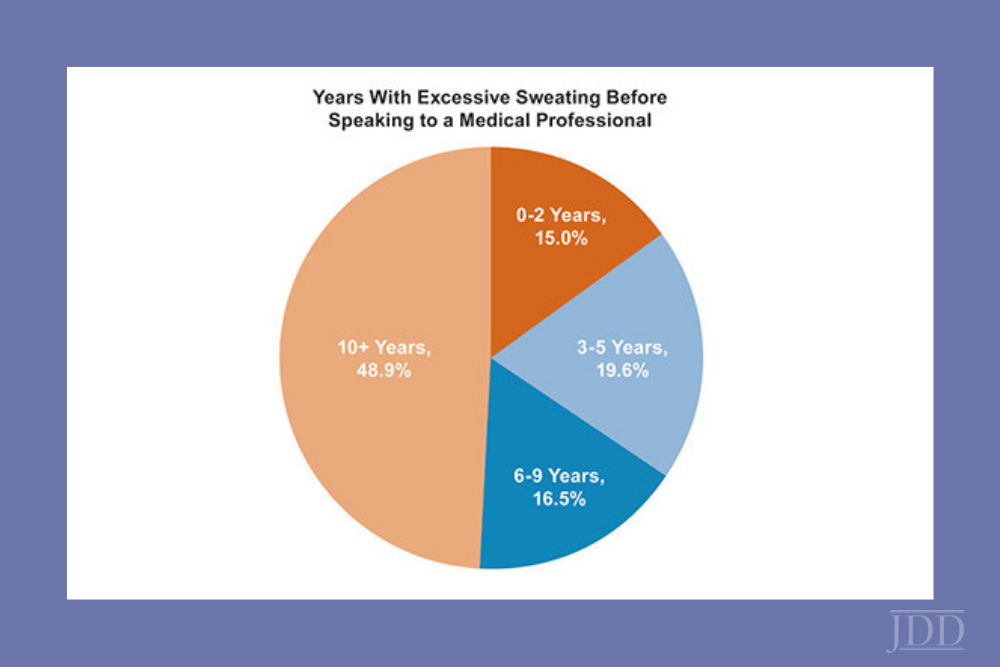
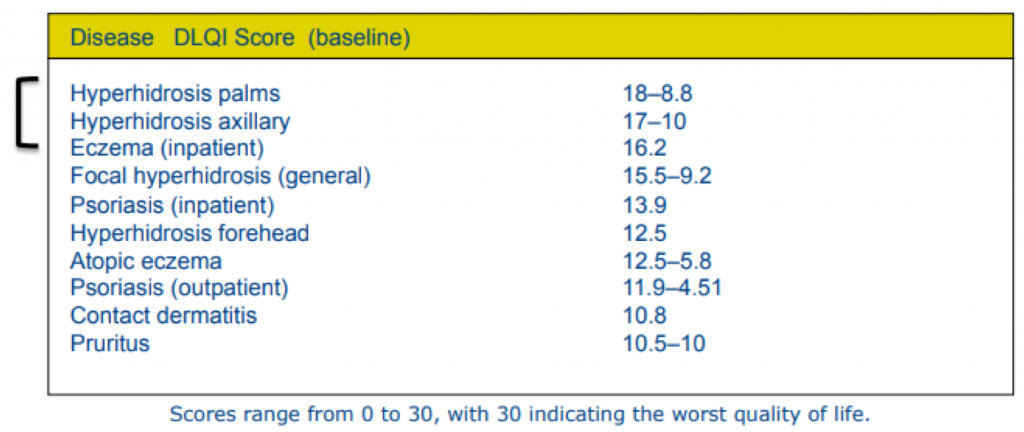
Source: ODAC Dermatology, Aesthetic & Surgical Conference (ODAC) Discovery in Dermatology Poster Session
At the 17th Annual ODAC Dermatology, Aesthetic, and Surgical Conference (ODAC) held January 17-20 in Orlando, FL, Brandon Kirsch, MD, Janet DuBois, MD, Martin N. Zaiac, MD and Deepak Chadha, MS, MBA, RAC presented scientific research of long term data with a novel therapeutic for topical treatment of primary axillary hyperhidrosis in pediatric subjects.
Discovery in Dermatology
The use of retro-metabolically designed drugs in dermatology is novel and has the potential for providing significant therapeutic benefit to pediatric and adult patients.
Sofpironium bromide is an ester analogue of glycopyrrolate that inhibits muscarinic receptors in sweat glands. It was developed according to the principles of retro-metabolic drug design, in which the goal is to create an active compound that is metabolized in vivo to an inactive moiety in a single, predictable reaction. Retro-metabolically designed drugs are rapidly metabolized in the bloodstream, potentially allowing for optimal therapeutic effect at application sites with minimal systemic side effects.
Introduction
~2.1% of the US population aged <18 years has primary hyperhidrosis (HH); ~65% have axillary HH. Long-term safety/tolerability and efficacy of topical HH treatments have rarely been studied in pediatric patients. Sofpironium bromide is a retro-metabolically designed analog of glycopyrrolate (anticholinergic) in development for topical treatment of primary axillary HH. Absorbed drug is rapidly metabolized, potentially allowing optimal local therapeutic effect with minimal systemic effects..
Procedures
21 of 25 subjects (age 9-16 yrs) with primary axillary HH of ≥6 months duration, completing a previous 1-week safety and pharmacokinetic (PK) study (BBI-4000-CL-105), were enrolled. Objectives were to assess safety/tolerability and PK, and explore efficacy of sofpironium bromide gel, 15% applied to both axillae for 24 weeks.
Results
Mean age (SD) 13.3 (2.29) years. 16 subjects completed this 24-week study. 7 had treatment emergent adverse events (TEAEs); 4 with AEs related to study drug, including expected systemic anticholinergic AEs (blurred vision, dry mouth, dry eyes, mydriasis) and local events (pain, pruritus, rash, erythema). 2 subjects discontinued due to TEAEs, including dry eye, dry mouth, local pruritus, local rash. The majority (52.4%) of subjects did not have any local symptoms/signs, and none observed were severe in nature. PK did not show evidence of drug/major metabolite accumulation, with most subjects having concentrations not quantifiable. The validated patient-reported outcome, Hyperhidrosis Disease Severity Measure-Axillary (HDSM-Ax), showed mean (SD) change from baseline (from previous study) to Week 24 of this study of -1.91 (1.038). A -1.00 change shows clinically meaningful improvement.
Conclusion
In this 24-week study in pediatric subjects sofpironium bromide, 15% was safe/well tolerated. Majority of subjects had no TEAE, and there were no severe or serious AEs. There was no evidence of drug accumulation. There was indication of clinically meaningful improvement in axillary HH.