ODAC Dermatology Conference, in partnership with Next Steps in Derm, interviewed Dr. David Miller, Instructor in Dermatology and Medicine at Harvard Medical School and member of the Department of Dermatology and the Department of Medicine at Massachusetts General Hospital, where he is co-director of the Merkel cell carcinoma treatment program. Watch as he shares important updates on Immuno-Oncology (IO) strategies for melanoma.

Source: Next Steps in Derm
Backed by a mountain of evidence, Dr. Zitelli walked us through the new and changing role of sentinel lymph node biopsy for melanoma in a riveting 20-minute talk presented at the 16th annual ODAC conference. Here are the highlights.
“Let’s separate what’s really evidence based from what you’ve been told.”
Before delving in, Dr. Zitelli skillfully laid the framework for his lecture. The crux of sentinel lymph node biopsy is based on the theory of orderly progression, in which malignant melanoma cells leave the tumor and preferentially enter the lymphatics and the first lymph node. This theory is rivaled by the anatomic pathway, in which malignant melanoma cells may enter the lymphatics or the blood stream, resulting in simultaneous dissemination.
Which theory is correct?
The overwhelming preponderance of evidence supports the latter anatomic theory – melanoma cells may enter the blood stream directly or the lymphatics, potentially bypassing the sentinel node. This anatomic theory is evidence based. It refutes the theory of orderly progression that the concept of sentinel lymph node biopsy is based on. Another common misconception is that lymph nodes are filters – they are not. Lymph nodes are sampling organs, sampling antigens in order to initiate an immune response.
With the groundwork laid, Dr. Zitelli went on to summarize the emerging evidence for sentinel lymph node biopsy. “This is what you need to know when you counsel a patient in order to obtain true informed consent.”
What you’ve been told: Sentinel lymph node biopsy improves survival
What the evidence shows: There is not a single solid tumor for which sentinel lymph node biopsy has been shown to provide a survival benefit.
We’ve been told that sentinel lymph node biopsy improves survival in intermediate thickness melanoma, because subclinical deposits are removed from the lymph nodes before they can grow. In fact, 33% of patients who underwent sentinel lymph node biopsy, did so because they thought it would improve their survival. Yet, there is not a single solid tumor – melanoma, gastric, renal, thyroid or otherwise – where electively removing normal lymph nodes, even in the case of microscopic involvement, has shown a survival benefit.
A cornerstone trial, the Multicenter Selective Lymphadenectomy Trial (MSLT-1), set out to prove the survival benefit of sentinel lymph node biopsy in melanoma. However, sentinel lymph node biopsy failed to improve melanoma specific survival. Subsequently, MSLT-2 looked at whether removing positive lymph nodes further down the line would improve survival in patients who had positive sentinel lymph nodes – this was also a negative study.
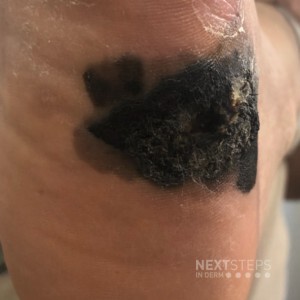
Source: Dermatology Times
Sentinel lymph node biopsy (SLNB) has classically been performed for regional disease control and to hopefully prevent disease metastasis; however, according to one expert, there has not been any good evidence to support this practice. As such, it is important for clinicians to focus on the evidence when planning the treatment and management of their advanced melanoma patients.
“Over the last decade or so, the role of SLNB has been changing, and there is no real consensus as to when to perform the procedure because it is a very rapidly changing field. The touted usefulness in survival benefit or prognosis of SLNB simply cannot be backed up by the available data, essentially rendering the appropriate use of SLNB in therapeutic limbo,” said John Zitelli, M.D., clinical associate professor, departments of dermatology & otolaryngology, University of Pittsburgh Medical Center, Pittsburgh, Penn., who spoke at the Orlando Dermatology and Aesthetic Conference.
According to Dr. Zitelli, the theory that SLNB would provide a survival benefit was debunked with the MSLT-1 research study1 recently published in the New England Journal of Medicine, and the idea that the procedure was to be considered as the most accurate prognostic test was also shown to be untrue. There usually is no need to do a SLNB, Dr. Zitelli said. The Breslow thickness, as well as all of the presenting clinical pathological morphologic features, such as ulceration of the tumor, is a wealth of information that the clinician can use to contemplate appropriate further treatment and management of the patient. Many clinicians still prefer to perform SLNB, Dr. Zitelli said, reasoning that waiting until the tumor is palpable would likely be synonymous with greater complications.
“The premise is off, because if you’re performing SLNB on a lot of people and the complication rate is low but the number of patients who are getting the procedure is high, the long-term complication rate in a group of people who you manage with SLNB actually have more complications than the smaller group of patients who have a complete node dissection from palpable disease,” Dr. Zitelli said.
Controversy revolving around the role of SLNB and its true usefulness in melanoma therapy and management continues today. The current contemporary wisdom is that SLNB should be performed because the results could help determine which patients would be more amenable to adjuvant therapy.
Source: Next Steps in Derm
This information was presented by Dr. Jean Bolognia at the 16th Annual ODAC Dermatology, Aesthetics and Surgical Conference held January 18th-21st, 2019 in Orlando, FL. The highlights from her lecture were written and compiled by Dr. Daniel Yanes.
Despite being one of the more common reasons for consulting a dermatologist, the diagnosis and management of atypical nevi remain nuanced and can often be challenging. I had the opportunity to learn from Dr. Jean Bolognia on her approach to atypical nevi, and walked away with many pearls to share.
1. Identify the patient’s signature nevus and come up with a plan.
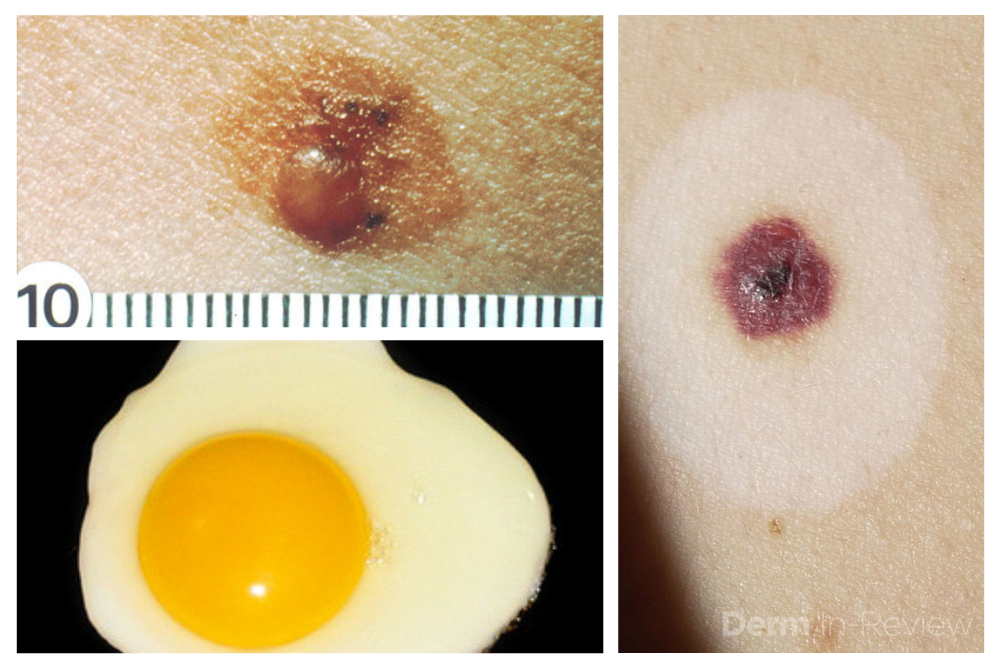
Sometimes it can be overwhelming to know where to begin when tasked with the patient who has numerous and atypical nevi. The first step is to identify the patient’s signature nevus. Do they tend to grow fried egg nevi, eclipse nevi, or cockade nevi? Are their signature moles all pink with little brown pigment, or are they pitch black with a wafer of scale? Identifying the signature nevus assists in determining the ugly duckling, and it will also help you develop a practical approach. In addition, if the patient has primarily pink nevi, palpation for induration versus soft flabbiness is helpful as banal intradermal melanocytic nevi can be pink in color. If the patient has primarily small flat black nevi, you should hone in on the presence of inflammation that is not simply due to acne or folliculitis. Creating an individualized plan is the key to a successful examination.
2. Nevi change, and sometimes it is simply an aging phenomenon.
In addition to identifying the signature nevus, it is also essential to understand how melanocytic nevi evolve over time. While nevi classically progress from junctional to compound and then to dermal, sometimes they simply fade away. In the case of fried egg nevi, the “yolk” becomes more raised and softer over time while the “white” of the egg gradually fades (figure 1). This results in multiple large dermal nevi on the trunk in an older patient. Patients can be taught that when a nevus elevates, determining if the lesion is firm versus soft can assist in distinguishing between the need for evaluation versus an aging phenomenon. Although not all changing nevi are concerning nevi, it is still essential to give the patient’s nevus of concern special attention, even if it doesn’t catch your eye at first.
Source: Next Steps in Derm
This information was presented by Dr. Jean Bolognia at the 16th Annual ODAC Dermatology, Aesthetics and Surgical Conference held January 18th-21st, 2019 in Orlando, FL. The highlights from her lecture were written and compiled by Dr. Daniel Yanes, one of the 5 residents selected to participate in the Sun Resident Career Mentorship Program (a program supported by an educational grant from Sun Pharmaceutical Industries, Inc.). Dr. Yanes was paired with Dr. Jean Bolognia as his mentor.
The world of melanoma is evolving, and dermatologists need to be equipped with the knowledge to help their patients navigate this landscape. Newer therapies for patients with more advanced stages of melanoma have not only drastically improved survival, but we as dermatologists must be prepared to recognize and treat the cutaneous side effects of these medications. This is a brief summary of common systemic therapies for melanoma with which every dermatologist should become familiar.
MAP Kinase Pathway Inhibitors
Selective BRAF Inhibitors
Melanoma tumor cells often have activating mutations that lead to constitutive activation of the MAP kinase pathway (See figure). Such activation can then lead to unregulated cell growth and proliferation. The most commonly detected mutation in BRAF results in the substitution of glutamic acid (E) for valine (V) at the 600th position in the BRAF protein and is referred to as BRAF V600E. Selective BRAF inhibitors, e.g. dabrafenib, encorafenib and vemurafenib, specifically target altered BRAF proteins. You can easily recognize these medications from their names, with raf indicating they target (B)RAF and nib identifying them as inhibitors. They are administered orally and chronically and lead to rapid responses but unfortunately tumor resistance commonly develops, often within six months. There are cutaneous side effects that the dermatologist should recognize, including morbilliform and folliculocentric eruptions, UVA photosensitivity (e.g. vemurafenib), keratoacanthomas/squamous cell carcinomas, and changes in melanocytic nevi (eruptive, enlargement, involution).
MEK Inhibitors
When mechanisms of resistance to selective BRAF inhibitors were investigated, a common finding was re-activation of the MAP kinase pathway via activation of MEK, another kinase that is downstream from BRAF. MEK inhibitors, e.g. binimetinib, cobimetinib, trametinib, were then combined with selective BRAF inhibitors to reduce the development of tumor resistance. These drugs are identified by the presence of a -metinib suffix. Interestingly, compared to BRAF inhibitors alone, combination BRAF+MEK therapy is associated with significantly less, not additive, cutaneous side effects – a real benefit to the patient.
Immunotherapy – Checkpoint Inhibitors
Immunotherapy is designed to stimulate the immune system to attack immunogenic melanoma cells. These monoclonal antibodies inhibit inhibitory signals that normally downregulate the immune system and thus act as immune checkpoints. These drugs model after the saying “the enemy of my enemy is my friend,” only it’s now “the inhibitor of the immune inhibitor is the immune stimulator.” CTLA4 is a receptor on regulatory T cells that plays an important role in diminishing immune responses. By blocking the inhibitory function of CTLA4 during the priming phase, the anti-CTLA4 antibody ipilimumab increases T cell immune activity. Peripherally, when the PD-1 receptor on T cells binds to its ligand, PD-L1, on tumor cells, an inhibitory signal results. In a similar fashion, the anti-PD-1 monoclonal antibodies approved for melanoma – nivolumab and pembrolizumab – can increase anti-tumor immune activity. Anti-PD-L1 monoclonal antibodies (e.g. avelumab, atezolizumab, durvalumab) have been approved to treat other malignancies, including Merkel cell carcinoma.

Source: Dermatology News
When caring for individuals with sun-damaged skin, dermatologists need comfort with the full spectrum of photo-related skin disease. From assessment and treatment of actinic keratoses (AKs) and field cancerization, to long-term follow-up of cutaneous squamous cell carcinomas (SCCs), appropriate treatment and staging can improve patient quality of life and reduce health care costs, Vishal Patel, MD, said at the Orlando Dermatology Aesthetic and Clinical Conference.
said Dr. Patel, director of cutaneous oncology at George Washington University Cancer Center, Washington. On the other hand, he added, “field disease can be a marker for invasive squamous cell carcinoma risk, and it requires field treatment.” Treatment that reduces field disease is primary prevention because it decreases the formation of invasive SCC, he noted.

Source: Dermatology News
Pearls for providers of photodynamic therapy (PDT) include tips on skin preparation, eye protection, and use of three new codes to maximize reimbursement. Also trending in medical dermatology are best practices for intralesional injections of 5-FU to treat the often challenging isomorphic squamous cell carcinomas (SCCs) or keratoacanthomas on the lower leg, as well as use of neoadjuvant hedgehog inhibitors to shrink large skin cancer lesions, according to Glenn David Goldman, MD.
“This talk is about what you can do medically as a dermatologic surgeon,” Dr. Goldman said at the Orlando Dermatology Aesthetic and Clinical Conference.
Use new billing codes for photodynamic therapy
There are now three new PDT billing codes. “Make sure your coders are using these properly. They are active now, and if you don’t use them, you won’t get paid properly,” said Dr. Goldman, professor and medical director of dermatology at the University of Vermont, Burlington. Specifically, 96567 is for standard PDT applied by staff; 96573 is for PDT applied by a physician; and 96574 is for PDT and curettage performed by a physician.
“Be involved, don’t delegate,” Dr. Goldman added. “If you do, you will get paid half as much as you used to, which means you will lose money on every single patient you treat.”
What type of PDT physicians choose to use in their practice remains controversial. “Do you do short-contact PDT, do you do daylight PDT? We’ve gone back and forth in our practice,” Dr. Goldman said. “I’m not impressed with daylight PDT. I know this is at odds with some of the people here, but at least in Vermont, it doesn’t work very well.”
The way PDT was described in the original trials (a photosensitizer applied in the office followed by PDT) “works the best, with one caveat,” Dr. Goldman said. The caveat is that dermatologists should aim for a PDT clearance that approaches the efficacy of 5-fluorouracil (5-FU). “If you can get to that – which is difficult by the way – I think your patients will really appreciate this.”
An additional PDT pearl Dr. Goldman shared involves skin preparation: the use of acetone to defat the skin, even in patients with very thick lesions. Apply acetone with gauze to the site for 5 minutes and “all of that hyperkeratosis just wipes away,” curette off any residual hyperkeratosis – and consider a ring anesthetic block to control pain for the patient with severe disease, he advised.
Another tip is to forgo the goggles that come with most PDT kits. Instead, purchase smaller, disposable laser eye shields for PDT patients, Dr. Goldman said. “They work better. You can get closer to the eye … and they are more comfortable for the patient.”
Dr. Goldman’s practice is providing more PDT and much less 5-FU for patient convenience. “I believe if someone is willing to go through 3 weeks of 5-FU or 12-16 weeks of imiquimod, they get the best results. However, most people don’t want to do that if they can sit in front of a light for 15 minutes.”
Consider intralesional injections for SCCs and KAs on the legs
An ongoing challenge in medical dermatology is preventing rapid recurrence of SCCs and/or keratoacanthomas (KAs) near sites of previous excision on the legs. “We all see this quite a bit. Often you get lesions on the leg, you cut them out, and they come right back” close to the excision site, Dr. Goldman said.
He does not recommend methotrexate injections for these lesions. “Methotrexate does not work. It doesn’t hurt, but I’ve injected methotrexate into squamous cell carcinomas many times and they’ve never gone away.” In contrast, 5-FU “works incredibly well. They go away, I’ve had tremendous success. This has changed the way we treat these lesions.” 5-FU is inexpensive and can be obtained from oncology pharmacies. One caveat is 5-FU injections can be painful and patients require anesthesia prior to injection.

Source: Dermatology Times
Options for repairing nasal defects after skin cancer surgery should be based on location, size and depth of the defect, as well as patient preference.
“If the defect is centrally located in the alar groove, you may want natural healing to occur,” says Joel L. Cohen, M.D., associate clinical professor of dermatology at the University of Colorado in Denver, and director of AboutSkin Dermatology in Greenwood Village and Lone Tree, Colo. He spoke with Dermatology Times prior to his presentation on skin cancer nasal reconstruction at the recent Orlando Dermatology Aesthetic & Clinical Conference (ODAC) in Miami.
“In such a case, the natural concavity is often recapitulated by simply letting the skin granulate, without the need for any sutured repair.”
However, in many instances of nasal reconstruction, dermatologists have to decide which procedure will achieve the best aesthetic outcome and also, the level of wound care that can be managed by the patient.

Source: Dermatology News
In his practice, Joel L. Cohen, MD, spends a good part of his day doing Mohs surgery, “with the goal of cancer removal, and after surgery, having the patient look good,” he said at the Orlando Dermatology Aesthetic and Clinical Conference.
“Having resurfacing in my practice has allowed me to treat not only wrinkles and etched lines, but also help skin cancer patients by blending and minimizing their skin cancer scars,” said Dr. Cohen, an aesthetic dermatologist and Mohs surgeon in private practice in Denver.
For example, one of his patients was a kindergarten teacher who had a large rotation flap scar on her cheek after excision of a melanoma in situ. The children asked her about it all the time during the 2 months after the surgery, and she decided to come in for some laser sessions. “With three ablative fractional laser sessions, she really looked great just 3 months later and wasn’t even interested in wearing makeup at that point.”
Resurfacing in his practice using a variety of lasers is very helpful, Dr. Cohen said. He published a study in November that compared pulse dye laser, CO2 ablative fractional lasers, or a combination of both for modification of scars following Mohs surgery (J Drugs Dermatol. 2016 Nov 1;15[11]:1315-9).
The prospective, multicenter study revealed that although both monotherapy approaches were safe and effective, the combination of pulse dye laser and fractional ablative laser offered some synergy that was preferred by patients.
